Published date: 12 September 2023
Authors: S. Sarkar,* A. Shil, S. Maity, Y. L. Jung, M. Dai, A. Acharya,* and K. H. Ahn*
Page link: https://onlinelibrary.wiley.com/doi/10.1002/anie.202311168
Graphical Abstract
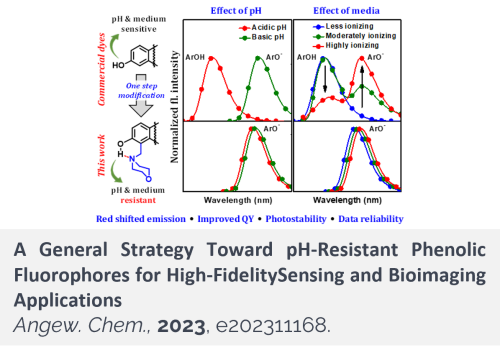
Due to the polar and acidic hydroxyl functionality, phenolic fluorophores provide pH and medium-dependent emission signals, which complicates bioimaging experimental design and reduces the data reliability. We developed a single-step functionalization that makes phenolic fluorophores pH and medium-resistant. The new fluorophores can be used for high-fidelity sensing and bioimaging applications.
Abstract
Aryl alcohol-type or phenolic fluorophores offer diverse opportunities for developing bioimaging agents and fluorescence probes. Due to the inherently acidic hydroxyl functionality, phenolic fluorophores provide pH-dependent emission signals. Therefore, except for developing pH probes, the pH-dependent nature of phenolic fluorophores should be considered in bioimaging applications but has been neglected. Here we show that a simple structural remedy converts conventional phenolic fluorophores into pH-resistant derivatives, which also offer “medium-resistant” emission properties. The structural modification involves a single-step introduction of a hydrogen-bonding acceptor such as morpholine nearby the phenolic hydroxyl group, which also leads to emission bathochromic shift, increased Stokes shift, enhanced photo-stability and stronger emission for several dyes. The strategy greatly expands the current fluorophores’ repertoire for reliable bioimaging applications, as demonstrated here with ratiometric imaging of cells and tissues.