Published date: 09 July 2023
Authors: S. Sarkar, A. Shil, Y. W. Jun, Y. J. Yang, W. Choi, S. Singha,* and K. H. Ahn*
Page link: https://onlinelibrary.wiley.com/doi/10.1002/adfm.202304507
Abstract
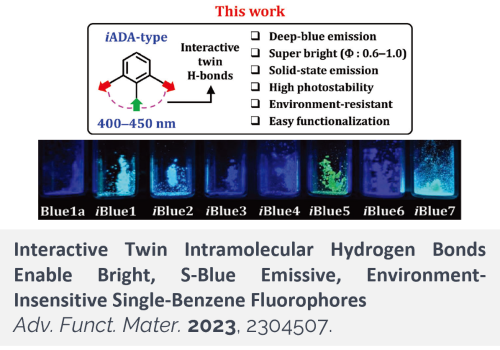
Small-molecule dyes that emit blue fluorescence, particularly those that emit in the S-blue region, are limited in number. Functionalization of benzene with electron-donating and -accepting groups generates single-benzene fluorophores (SBFs) with tunable emission properties. By exploring the unprecedented, interactive twin intramolecular H-bonds, a novel type of SBFs that exhibits several notable features for bioimaging applications is developed. Specifically, aniline derivatives bearing two carbonyl/carboxyl groups at ortho-positions have interactive H-bonds, which lead to conformational restriction, suppressed solvation, and increased HOMO-LUMO energy gaps from those of the conventional SBFs that have non-interactive H-bonds. The interactive H-bonds are manifested by notable photophysical properties among the new SBFs, such as S-blue emissive, bright, photo- and chemo-stable, solid-state-emissive, and environment (pH, polarity, and viscosity)-insensitive. The use of the highly faceted dye system to develop a reliable fluorescent probe, organelle-staining dyes, and a fluorescence-resonance-energy-transfer probe for hydrogen sulfide demonstrates its potential in bioimaging applications.